In Canada, Health Canada sets specific regulatory requirements for “supplemented foods.” These products are gaining popularity, from energy drinks and fortified snacks to protein bars with added vitamins or minerals. However, with increased popularity comes the need for clear supplemented labelling and compliance with Canadian food laws.
If you’re a food manufacturer, distributor, or importer, understanding what “supplemented” means, the rules that apply, and how to create a supplemented Health Canada label is essential. This guide explains everything, from the supplemented definition to Health Canada’s labelling rules, so your products remain compliant.
Key Takeaways
- Supplemented foods are everyday foods with added supplemental ingredients (e.g., vitamins, minerals, amino acids, caffeine).
- Health Canada requires specific labelling elements for supplemented foods, including the Supplemented Food Facts table.
- Compliance involves following Canada FDA regulations (CFIA & Health Canada).
- Businesses can use nutrition label software, like MenuSano, to generate fully compliant supplemented labels quickly.
What Does “Supplemented” Mean?
In Health Canada’s terms, supplemented foods are foods and beverages with added supplemental ingredients that are not generally found in that type of food or are present in higher amounts than expected.
Examples of supplemental ingredients include:
- Vitamins and minerals beyond the naturally occurring amounts
- Amino acids (e.g., taurine)
- Caffeine
- Herbal ingredients (e.g., ginseng, guarana)
The supplemented definition is crucial because it determines whether your product falls under standard food labelling rules or the supplemented foods regulations.
Supplemented Foods and Health Canada
When you see the phrase “supplemented Health Canada”, it refers to foods regulated under the new supplemented foods framework introduced in 2022. These rules are part of Health Canada’s effort to protect consumers by ensuring that products with added functional ingredients are safe, properly labelled, and clearly understood by the public.
Health Canada’s Supplemented Foods Regulations
The Canada CFIA regulations (commonly referred to as Health Canada and the CFIA) outline the following requirements for supplemented foods:
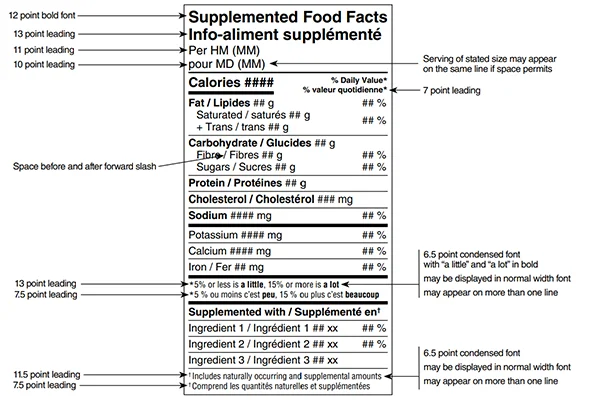
- Supplemented Food Facts Table (SFFT): This modified version of the standard Nutrition Facts table shows added supplemental ingredients alongside regular nutrient information.
- List of Supplemental Ingredients – Must be clearly identified in the ingredient list.
- Cautionary Statements – If a food could pose a health risk to certain groups (e.g., children, pregnant women, people sensitive to caffeine), the label must include clear warnings.
- Daily Maximum Amounts – Health Canada sets limits for supplemental ingredients to prevent excessive consumption.
- Standardized Labelling Format – All supplemented foods must use the CFIA-compliant design for placement, font size, and legibility.
Examples of Supplemented Foods in Canada

- Energy drinks with added caffeine and B vitamins
- Breakfast cereals fortified with extra minerals and protein
- Snack bars with added amino acids
- Sports beverages with electrolytes and herbal extracts
- Plant-based milks with additional nutrients beyond standard fortification
These additional foods must follow the supplemented regulations if they contain supplemental ingredients above normal dietary levels.
Why Supplemented Food Label Compliance Matters
Selling a supplemented food without proper labelling can have serious consequences for a business. Improper or missing information can trigger product recalls, cause costly delays at import or export checkpoints, and even lead to fines or other legal penalties. Beyond the legal risks, it can also damage a company’s reputation and erode consumer trust, which is often far harder to rebuild than to lose.
By complying with supplemented food labelling requirements, businesses ensure their products meet Health Canada’s standards while providing clear and accurate information to consumers. Proper compliance not only helps avoid regulatory issues but also reinforces credibility in the marketplace, giving customers confidence in the safety and quality of the products they purchase.
How to Create a Supplemented Health Canada Label
- Identify supplemental ingredients in your product.
- Check Health Canada’s permitted levels for each ingredient.
- Determine if your product needs a Supplemented Food Facts Table.
- Include mandatory cautionary statements if required.
- Format your label according to CFIA design standards.
Using nutrition label software like MenuSano can save time, eliminate guesswork, and ensure your supplemented food labels are accurate and compliant. Here’s a detailed guide on how to create supplemented food facts labels.
How MenuSano Can Help
Manually creating a supplemented Health Canada label can be complex. MenuSano’s nutrition analysis software:
- Automatically generates CFIA-compliant Supplemented Food Facts tables
- Ensures correct formatting, font sizes, and placement
- Calculates and displays all required supplemental ingredients
- Helps you stay up-to-date with Canada’s FDA regulations
This means you can focus on developing your products while knowing your labels meet Health Canada’s standards.
The regulations for supplemented foods in Canada are designed to protect consumers and create transparency in the marketplace. Whether you produce energy drinks, fortified snacks, or functional beverages, understanding supplemented Health Canada meaning is key to staying compliant.
By following the Canada FDA regulations and using tools like MenuSano, you can confidently create supplemented food labels that meet every requirement. This will help you avoid penalties, speed up product approvals, and build trust with your customers.






















