As Canada continues to evolve in its legal cannabis framework, understanding and adhering to cannabis packaging and labelling regulations is not optional; it’s the law. Health Canada, under the Cannabis Act (SOR/2018-144), has established comprehensive rules for all cannabis products to ensure public health and safety, prevent appeal to youth, and support informed consumer choices.
Whether you’re a licensed cultivator, processor, distributor, or retailer, complying with the Cannabis Regulations’ packaging and labelling requirements is crucial to avoid costly recalls, penalties, or legal complications. This guide will help you understand the ins and outs of CFIA-compliant cannabis packaging and labelling in Canada.
Key Takeaways
- Health Canada’s cannabis packaging and labelling requirements are mandatory for all licensed producers and sellers.
- Cannabis logo placement, THC/CBD content, and health warnings are strictly regulated.
- Packaging must be plain, child-resistant, and tamper-evident with no promotional elements.
- Non-compliance can result in fines, recalls, or suspension of licenses.
- Tools like MenuSano can help generate CFIA- and Cannabis Act-compliant labels efficiently.
Understanding Canada’s Cannabis Packaging Regulations
In Canada, cannabis packaging is designed to inform and protect consumers rather than to promote products. Every package must follow standardized rules covering appearance, typography, health warnings, and mandatory information. Unlike many industries where branding dominates packaging design, the Canadian cannabis market enforces plain packaging with limited use of brand elements.
The Cannabis logo—a red stop-sign shape featuring a cannabis leaf and the letters “THC”—is one of the most recognizable and regulated elements on cannabis packaging. It must appear on any product containing THC above 10 micrograms per package. The logo serves as a clear visual warning to consumers, particularly to prevent accidental consumption by youth or non-users. Placement, size, and colour specifications for the logo are defined in the Cannabis Regulations, and failure to meet them can lead to enforcement action.
Key Elements Required on Cannabis Labels
Every cannabis product sold in Canada must feature core mandatory information, presented in a legible, bilingual format (English and French), with standardized formatting and terminology.
1. Brand Name and Product Name
- The brand name is permitted but must not include false, misleading, or promotional elements.
- The product name must accurately reflect the product’s type and contents.
2. THC and CBD Content
All cannabis labels must provide accurate, bilingual information in both English and French, ensuring accessibility to all Canadian consumers. Among the required elements are the brand name, the product name, and detailed cannabinoid content, including THC and CBD levels in both “total” and “non-activated” forms. Depending on the product type, these values must be precise and expressed in the proper units (mg/g or mg/unit).
3. Standardized Cannabis Symbol

- Required on all products containing THC, except products with no more than 10 micrograms of THC per package.
- Must appear prominently in the top left corner of the principal display panel.
- Must not be smaller than 1.27 cm x 1.27 cm.
4. Health Warning Messages
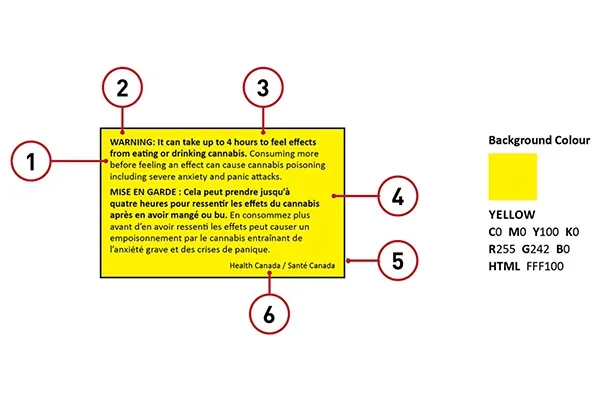
Source: Health Canada
A health warning message is also mandatory and must be displayed prominently. These warnings are standardized by Health Canada and rotate among approved statements to ensure consumers are informed of potential health risks.
- Must be bilingual
- Placed on the principal display panel
- Must use black text on a yellow background inside a rectangular border
- Font size must be a minimum of 6 points
- Example: “WARNING: Frequent and prolonged use of cannabis containing THC can contribute to mental health problems over time.”
Mandatory Content
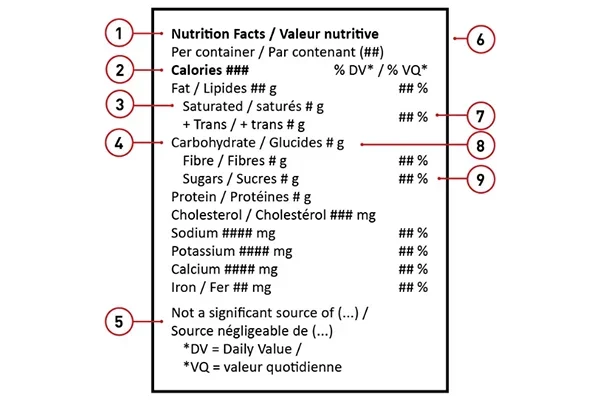
Additionally, packaging must include the packaging date, lot number, and expiry date where applicable. Products such as edibles and topicals require an ingredient list and allergen declarations, while edibles must also feature a Nutrition Facts Table that complies with CFIA standards. Storage instructions and usage guidelines are also required when relevant, especially for extracts and topicals.
- Packaging Date: The date the cannabis product was packaged must be listed in YYYY-MM-DD format.
- Lot Number and Expiry Date
- Lot number is required for product tracking and recalls.
- Expiry date is mandatory for edibles, topicals, and extracts.
- Net Weight or Volume
- For dried cannabis: expressed in grams (g)
- For liquids or oils: in millilitres (mL)
- Accuracy is critical: must match the actual content within specified tolerances.
- Storage Instructions: Labels must include recommended storage conditions, e.g., “Store in a cool, dry place.”
- Ingredients List
- Mandatory for edibles, topicals, and extracts
- Must list each ingredient in descending order by weight
- Include any allergens using bold text
- Intended Use and Directions
- Especially required for topicals and extracts
- Must state how to use the product and dosage guidance, if applicable
Design and Formatting Rules
Source: Health Canada
The visual design of cannabis packaging in Canada is strictly controlled. Bright colours, metallic or fluorescent finishes, and images that could appeal to youth are prohibited. Fonts must be plain and legible, with minimum size requirements to ensure readability. The entire approach prioritizes clarity over creativity.
Font and Size
- Use sans-serif font
- Minimum type size: 6 points
- All information must be clearly legible
Child-Resistant and Tamper-Evident
The packaging must also be child-resistant and tamper-evident, meeting standards set by the Canadian Standards Association (CSA Z76.1). This ensures that children cannot easily access the contents and that consumers can easily detect if a package has been opened before purchase.
No Promotional Content
The label must not contain:
- Testimonials or endorsements
- Lifestyle branding
- Celebrities or characters
- Claims of health or cosmetic benefits
- Comparisons with other products
Product-Specific Requirements
Different classes of cannabis products have additional requirements.
For dried and fresh cannabis, moisture content must be disclosed, and pre-rolls must include the number of units and weight per unit.
Cannabis extracts require details on carrier oils, solvent residues, and THC/CBD per activation.
Edible cannabis products must be clearly identified as cannabis-infused, provide nutritional information, and display THC/CBD amounts per serving and per package.
Topicals must include specific directions for use, warnings for external application, and ingredient listings following INCI standards when relevant.
Common Labelling Mistakes to Avoid
- Using non-compliant fonts or colours
- Incorrect THC/CBD calculations
- Missing or improper health warnings
- Overstating product effects or benefits
- Inaccurate or vague ingredient lists
- Including unauthorized images or branding
Enforcement and Penalties for Non-Compliance
Failure to comply with Canada’s cannabis packaging and labelling rules can have serious consequences. Health Canada conducts regular inspections and may issue recalls, fines, or administrative monetary penalties for violations. In severe cases, non-compliance can lead to license suspension or revocation. Public warnings and damage to brand reputation can further impact a company’s market position.
How to Ensure Cannabis Label Compliance in Canada
Given the complexity of cannabis labelling regulations, using specialized compliance tools can save businesses time, money, and legal headaches. Platforms like MenuSano provide the ability to create fully compliant labels that meet both CFIA and Cannabis Act requirements. By using software designed to handle the technical details, such as bilingual formatting, correct placement of the cannabis logo, and precise THC/CBD calculations, producers can focus on product quality while ensuring regulatory adherence.
Cannabis packaging and labelling in Canada is a highly regulated process that leaves no room for error. From the Cannabis logo to THC/CBD content declarations and health warnings, every detail is governed by Health Canada to protect consumers and prevent appeal to youth. Businesses that take compliance seriously not only avoid penalties but also build trust in an increasingly competitive market.
For companies seeking to streamline this process, MenuSano offers powerful labelling tools that make it easy to generate cannabis labels that comply with Canadian regulations, including all CFIA and Cannabis Act requirements. By combining accuracy, speed, and regulatory expertise, businesses can ensure their products reach consumers with confidence and compliance built in.






















