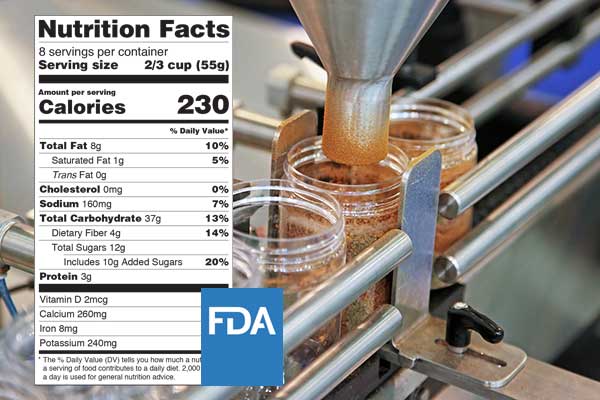
Foods that are sold to the public need not only be safe and wholesome but properly labelled. Food that’s produced domestically and internationally need to follow laws and regulations on food labels. The FDA governs food products and requires specific statements that must appear on food labels.
Failure to follow these guidelines can result in legal actions and delays in distributing your products. Using nutrition analysis software allows you to minimize disruptions in releasing your products and avoid any legal issues. This type of software lets you formulate and print out accurate nutrition labels in-house, which saves you time and money.
Food Labelling Requirements
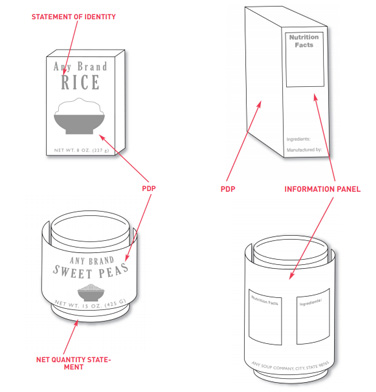
Label statements need to be placed in specific locations on containers and packages. There are two options where these labels need to be placed.
The first is to put them on the front label panel of the product. The second option is to place certain label information on the principal display panel and other labelling on the information panel.
The principal display panel or PDP is the main front label of the product if you are looking at it head-on. The information panel is immediate to the right of the PDP if the consumer is facing the product. If that’s not available due to packaging design, the information panel is then the next label panel immediately to the right.
The PDP is important for it is the statement of the identity of the product and it is also most likely to be seen by the customer. On this PDP, you are required to have the name of your food and the quantity or amount contained in it.
The Name of the Food

The food statement or statement of identity is simply the name of the food. It is required to appear on the front label or PDP. This is a prominent display and it should have bold print, and type to stand out. The type size needs to be reasonably related to the other prominent printed information on the front panel and be at least ½ the size of the largest print on the label.
The food statement needs to be focused on what the food is without being misleading. The purpose of this regulation is to describe the particular food. Brand names are not considered as statements of identity. The brand name should not stand out when compared to the statement of identity.
When it comes to imitation food, it must be labelled as such when it is a new food that resembles a traditional one. If it is a substitute for traditional food and contains less protein or any essential nutrient than the original, it must be labelled as ‘imitation’.
Contents Statements
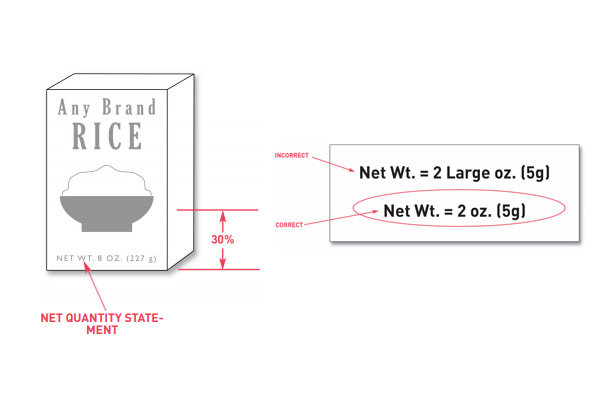
Net quantity needs to be included in your nutrition facts label. Net quantity is the statement on the label of the amount of food in one container or package. This measurement needs to be expressed in weight, measure, or the numeric count.
For products that are solid, semisolid, or viscous, the measurement should be expressed in weight. If the product is liquid, it needs to be expressed in a fluid measure.
For placement on the label, net quantity needs to be placed as a distinct item. It needs to appear in the bottom 30% of the principal display label and be parallel with the lines of the bottom of the container.
When printing net quantity statements on the packaging, qualifying statements should not be used. You shouldn’t use the terms that would exaggerate the amount of food either. An example of this is stating that the net weight of a product is 2 ‘large’ ounces. To correctly follow this policy, you would state that the net weight is simply 2 ounces.
Ingredient Lists Defined
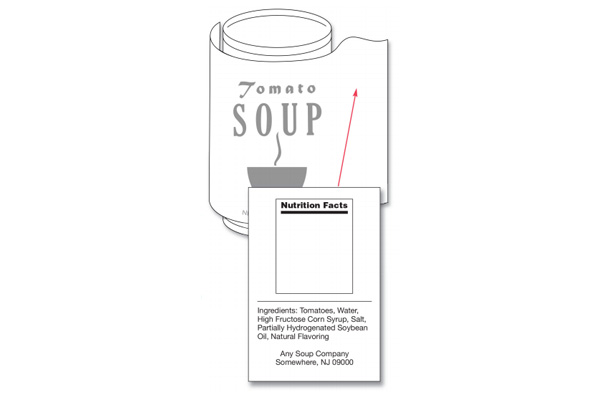
Having an accurate ingredient list is mandatory for any food product. The ingredients on a food label need to be listed in descending order of prominence. This means that the ingredient that weighs the most is listed first and so on, with the lightest ingredient listed last. The list of ingredients can be listed on the information panel or the PDP and needs to be on the same panel as the name and address of the manufacturer, packer, or distributor.
Common names need to always be listed in the ingredients. For example, you should list the term “sugar” instead of “sucrose”. Ingredients that may exist in trace amounts do not need to be listed, nor do ones that exist at an incidental level. This differs when it comes to allergens, however, as major food allergens must be listed even if they exist in trace amounts.
Major food allergens are one of the following or an ingredient that consists of protein derived from one of these:
- milk
- eggs
- fish
- crustacean shellfish
- tree nuts
- wheat
- peanuts
- soybeans
Listing possible allergens became regulation in 2004 with The Food Allergen Labelling and Consumer Protection Act. This protection act has identified 160 different possible food allergens, but the major food ones account for 90 percent of all food allergies.
When listing an alternate name of a major food allergen, the common name needs to be listed in parentheses. An example of this is a product that uses lecithin, for it must be stated as “(soy)”. Another way to do this is to include a “contains” section after the list of ingredients to display any major food allergens.
Different ingredients are used for colouring foods but still need to be listed in the label ingredients. Vegetable powders, spices, and artificial colours can change the colour of foods and need to be listed in the ingredients. Certified colour additives, including their lakes, are considered separate ingredients and must be declared independently in the ingredient statement.
Nutrition Labelling
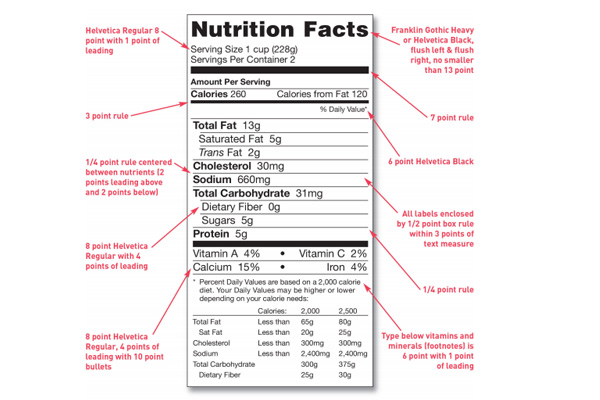
A nutrition facts label may be placed together with the ingredients list on the PDP. This label can also be placed on the information panel, or if there isn’t sufficient space, on the next panel to the right.
The nutrition facts labels need to be presented in a box format no matter what type of packaging it appears on. The product name cannot be placed in this box but it can be above the box that surrounds the nutrition information. The FDA’s goal is to have manufacturers present nutrition facts labels consistently so that it is readily observable for the consumer at the point of purchase.
Nutrient declarations need to be included on most food packages that are labelled. However, there are some exemptions. Some examples of this include food delivered to homes, bakery products, fresh produce, seafood, and foods that provide no significant nutrition.
Bulk foods present a challenge as the number of servings per container can be listed as “varied”. If the consumer is assumed to eat a combination of the packed products then a single composite analysis may be used to determine the nutrient composition.
Claims
Nutrient content claims need to be presented because it directly or by implication characterizes the level of a nutrient in any food. Examples of this include “low fat” or “contains 100 calories”.
For health claims, this is any claim on a label that characterizes the relationship of any substance to a disease or health-related condition. These claims are limited to disease risk reduction. Health claims cannot be claims about the diagnosis, treatment, or cure of any disease.
Any health claim must be evaluated by the FDA before use. When a claim is authorized, the label can then reflect that. Using soluble fibre as an example, the health claim can now mention how three grams of soluble fibre from oatmeal may help in reducing heart disease in a diet low in saturated fat and cholesterol.
Structure/function claims, on the other hand, do not mention diseases but characterizes the effect that a substance has on the structure or capacity of the body. An example of a structure/function claim is “strong bones are built by calcium.” These S/F claims must be true, cannot mislead, and are not pre-reviewed or approved by the FDA.
A qualified health claim helps educate consumers by providing them with more information on food labels concerning health and diet. The Consumer Health Information for Better Nutrition Initiative looks to provide interim procedures whereby qualified health claims are made available for both dietary supplements and conventional foods.
Conclusion
Nutrition facts labels present essential data that is needed to fulfill a healthy diet. There are so many areas that need to be considered and the MenuSano team understands that nothing can be left to chance. MenuSano is a nutrition analysis software that takes away any of the guesswork.
With our nutrition analysis software, you can easily create your nutrition labels. MenuSano is an affordable online solution to cater to your business needs. The government aims to keep its population healthy and foodservice providers now have all these regulations to follow. MenuSano provides you with the right tools to appease your health-conscious consumers.
Your nutrition labels can even be printed in house to move the process along quicker. When creating these labels, you can simply enter a recipe’s ingredients and their quantity, assemble the recipes into dishes, and download and print your new nutrition labels. MenuSano allows your business to comply with regulation standards so you can focus on delivering healthy food creations to your ever-growing customer base.

















